AstraBio’s plasma p-tau217 assay Gains China Regulatory Approval
AstraBio’s plasma p-tau217 assay Gains China Regulatory Approval
AstraBio is proud to announce that its plasma Phosphorylated tau-217 (p-tau217) Immune Test Kit has officially received Class II medical device registration approval. As a cutting-edge product based on AstraBio’s proprietary single-molecule immunoassay platform, the plasma p-tau217assay kit enables fg/mL-level ultra-sensitive detection from a minimal blood sample, offering a high performance, convenient and fully automated solution for early screening of Alzheimer’s disease (AD) and diagnosis with in clinical practice.
Uniquely, AstraBio’s P-tau 217 Immune Test kit is the only AD blood biomarker assay in China to have undergone rigorous validation across multiple cohort in Chinese population, including community-based populations, clinical memory clinics, and differential diagnosis cohorts. Key findings from these studies have been published in high-impact journals.
In collaboration with leading public hospitals and research insitition in China, AstraBio led one of the largest biomarker cohort studies in the country. The results showed that p-tau217 alone could distinguish healthy controls from aMCI and AD patients with 95% accuracy. The biomarker has also demonstrated diagnostic utility in other neurological disorders, further reinforcing its clinical versatility.
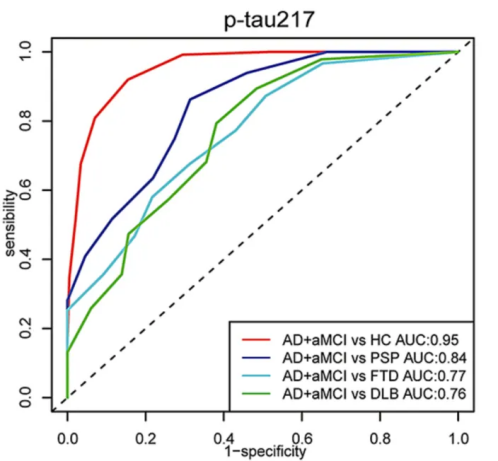
These findings indicate that AstraBio's P-tau 217 Immune Test kit not only meets the requirements of both domestic and international clinical guidelines(including recent publised, Alzheimer’s Association Clinical Practice Guideline: Integrating Blood-Based Biomarkers in Alzheimer’s Disease Specialty Care) and expert consensus, but also demonstrates high sensitivity and accuracy, providing a solid foundation for clinical application. It serves as a reliable tool for the accurate diagnosis and early detection of Alzheimer’s disease (AD) in China.
AstraBio’s Neuroscience Market Strategy
AstraBio has developed a leading single-molecule immunoassay technology with fully independent intellectual property rights. With an ultrasensitive detection limit in the fg/mL range, it can detect even minute changes in p-tau biomarkers in blood. The fully automated system delivers results in just 10 minutes, greatly reducing the clinical burden in terms of time, manpower, and resources—enabling fast and accurate AD diagnostics.
AstraBio is also establishing itself as a leading academic voice in the field of blood-based biomarkers for Alzheimer’s disease in China. With a neuroscience-focused pipeline, the company is driving the clinical translation and commercialization of novel biomarkers. AstraBio has formed deep collaborations with top-tier hospitals across China, and has published numerous high-impact scientific papers.
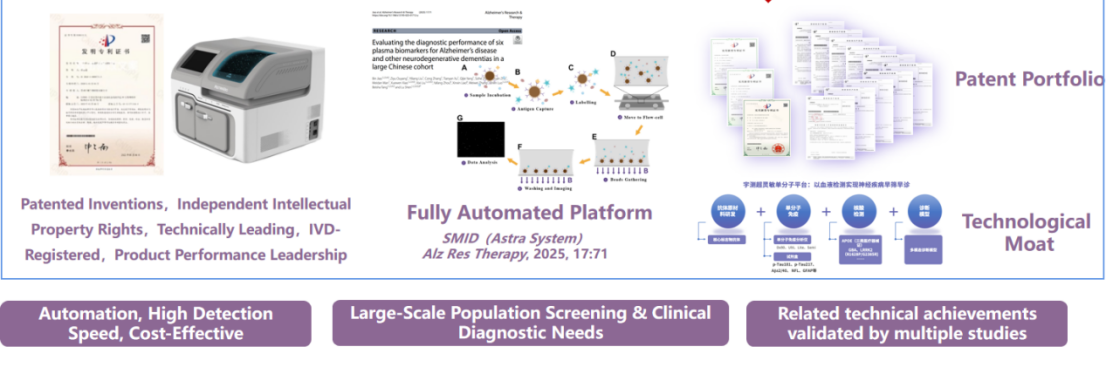
To date:
- Over 20,000 blood samples have been studied in advanced research and clinical centers.
- AstraBio has participated in multiple large-scale national cohort studies.
- The platform has contributed to publications with a cumulative impact factor exceeding 230, providing robust support for cutting-edge research on ultra-sensitive blood biomarkers in neuroscience.